
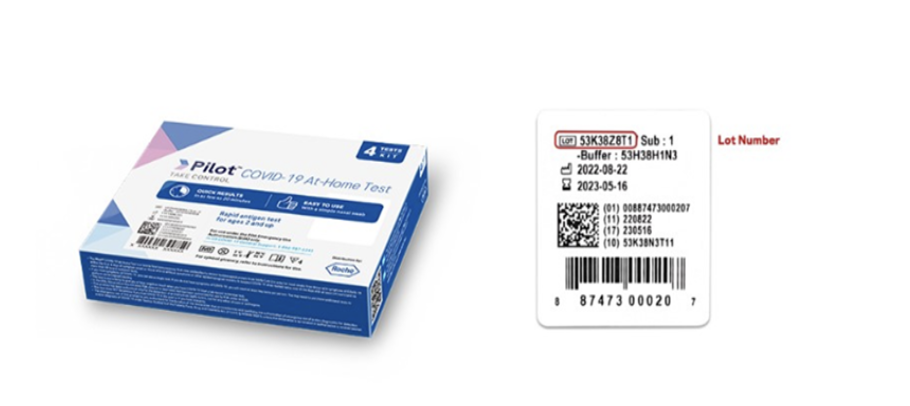
The US Food and Drug Administration has issued a safety communication concerning the SD Biosensor Inc. Pilot COVID-19 At-Home Tests, distributed by Roche Diagnostics. There are concerns of bacterial contamination in the liquid solution provided in the test kit. Direct contact with the contaminated liquid solution may pose safety concerns and the bacterial contamination could impact the performance of the test. Specific lot numbers of the test kits have been identified.
SD Biosensor Inc has issued a voluntary recall for the contaminated test kits. It is recommended that those in possession of a contaminated kit throw it in the household trash and not pour the test liquid down the drain.SD Biosensor Inc has set up a website for individuals to check to see if their kit is part of the contaminated lots and to order a free replacement kit if necessary. The SD Biosensor Inc Voluntary Recall information may be accessed at Pilot COVID-19 At-Home Test Voluntary Recall (roche.com).

